-
article · 2026Year57Moon4Day
Opentrons Flex vs 其他自动化 NGS 工作站对比评测
Read More -
article · 2026Year52Moon4Day
如何通过 Opentrons Protocol Library 运行 NGS 流程?
Read More -
article · 2026Year28Moon3Day
Opentrons Flex 的节省成本优势与投资回报分析
Read More


Author
BO person Lin,PhD,kin that day Watson,PhD 1open TR ONS lab works,Inc.
AbstractThis test investigated magnetic bead-based immobilized metal affinity chromatography (IMAC) on an Opentrons OT-2 automated pipetting workstation. We tested this protocol using PierceTM Ni-NTA magnetic agarose beads (Thermo Fisher Scientific, USA) for extraction of the target protein (His-tagged GAPDH) on OT-2, using recombinant GAPDH protein diluted in lysis buffer , HEp-2 cell lysate and samples prepared from bacterial cells.
Key Findings Ni-NTA magnetic beads can be used for purification of recombinant GAPDH protein on the Opentrons Protein Purification Workstation. ■ Experimental results prove that the protein obtained is the target protein with good consistency and specificity. ■ iThe solution can handle up to 96 samples and minimizes the time required for manual work.
IntroductionThe purpose of protein purification is to obtain high-purity, stable and active proteins for downstream analysis, research or therapeutic use. Immobilized metal affinity chromatography (IMAC) uses metal ions to extract recombinant proteins with genetic engineering tags, which can chelate the peptide chains of metal ions. Nickel-triethanolamine tetraacetic acid (Ni-NTA) coupled to agarose resin or magnetic beads is a commonly used IMAC tool for the purification of polyhistidine (His)-tagged proteins. Since the His residue can integrate nickel ions (Ni?+), His-tagged proteins show strong affinity to the Ni2*-immobilized carrier. His-tagged GAPDH was extracted using Pierce Ni-NTA agarose beads on an Opentrons Protein Purification Workstation.
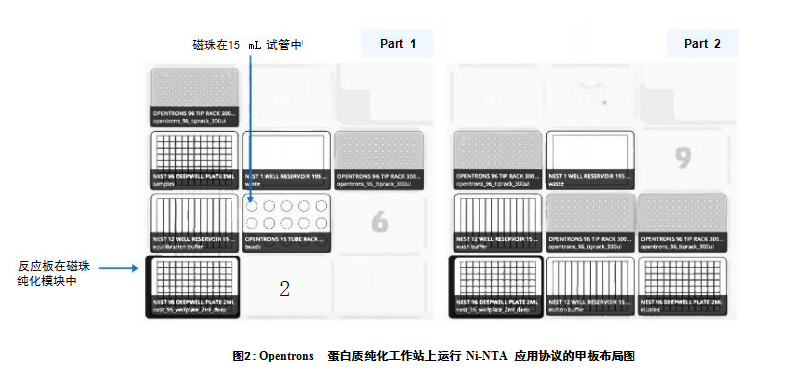
Materials and Methods Overview of the experimental process of IMAC Protocol The process of IMAC protocol is divided into several parts: Part 1: Preparation of sample/magnetic bead mixture. The target acquisition part is not performed on the OT-2 platform. Part II: Washing and Elution. The specific process is shown in Figure 1. Parts 1 and 2 are both performed on the OT-2. For 96 samples, the first part of the run took 57 minutes, followed by 30 minutes of target capture on a shaker. The second part takes 78 minutes to complete.
Ni-NTA experimental process on OT-2 platform The equipment layout diagram of the first and second parts is as follows (see Figure 2). Parts 1 and 2 of the protocol were both performed on the OT-2 platform and used the Magnetic Bead Purification Module to separate the magnetic beads from the solution. During sample processing, 500 μL of human His-tagged GAPDH solution was mixed with 12.5 μL of precipitated magnetic beads and incubated at room temperature at 800 rpm for 30 minutes. After 2 wash steps, the target protein is eluted in 250 μL elution buffer at 800 rpm and room temperature for 10 minutes on a shaker. 20 μL of the eluate was subjected to SDS-PAGE and Western Blot analysis, using monoclonal anti-GAPDH antibody (Thermo Fisher Scientific, USA) and secondary antibodies conjugated to fluorescent dyes (LI-COR Biosciences, USA) for detection.

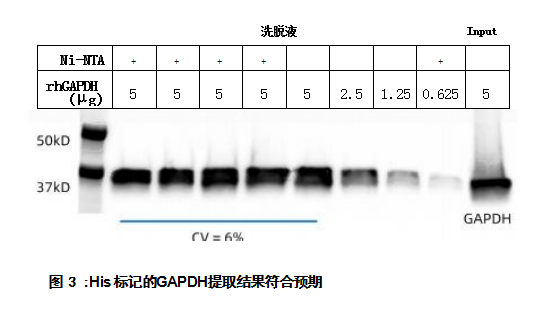
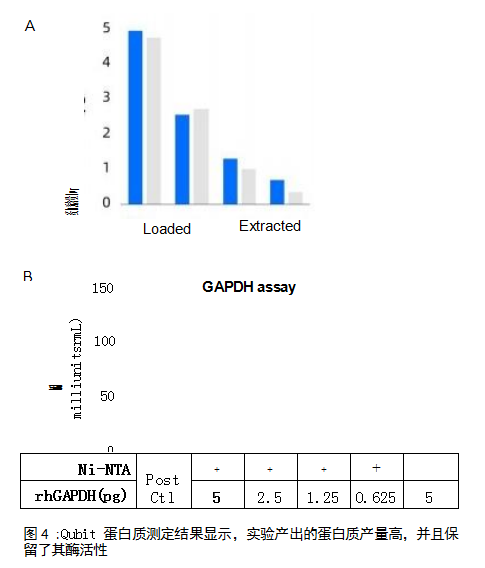
ResultsGAPDH was extracted in repeated experiments and obtained consistent sample processing results (protein band signal determined by Western blot analysis, CV=6%) (Figure 3). Part of the extract was quantified using the Qubit™ Protein Assay (ThermoFisher Scientific, USA), demonstrating high protein yield (Figure 4A). In addition, the protein purification process using the Opentrons Protein Purification Workstation does not destroy the protein's enzymatic activity, as measured by the GAPDH activity assay (Sigma-Aldrich, St. Louis, MO, USA) (Figure 4B).
Ni-NTA-mediated knockdown experiments are useful for isolating exogenously expressed His-tagged proteins in cell or tissue samples. The previous protocol was used to purify His-tagged GAPDH from 200 μL of HEp-2 cell lysate. The results once again demonstrated that His-GAPDH was successfully extracted and that the affinity specificity of Ni-NTA magnetic beads was very high compared to the binding of non-tagged proteins (Figure 5). In large-scale protein production, E. coli is commonly used for recombinant protein expression. Cell lysates were prepared by lysing bacterial cells with or without IPTG-induced expression of His-tagged GAPDH. The results further confirmed that this protocol was successful in purifying His-tagged proteins using Ni-NTA magnetic beads (Figure 6). 96 samples were processed in this study and the results shown are only for the 8 samples in the last column of the 96-well sample plate. At the same time, the experimental results also evaluate the running time of different sample sizes (Figure 7).
Conclusion In this study, an automated experimental workflow solution for the purification of His-tagged recombinant GAPDH protein based on magnetic beads was run on the Opentrons protein purification workstation. Experiments have proven that the Opentrons Protein Purification Workstation can process samples stably and efficiently in medium to high-throughput settings, reducing operation time while providing expected reproducible output.
Original address:Using Opentrons protein purification workstation based on Ni-NTA Protein Purification with Magnetic Beads
The experienced service team and strong production support team provide customers with worry-free order services.

简体中文

繁體中文

English

日本語

한국인